45 exempt human specimen examples
grants.nih.gov › policy › clinical-trialsNIH's Definition of a Clinical Trial | grants.nih.gov Aug 08, 2017 · NIH Definition of a Clinical Trial. A research study in which one or more human subjects are prospectively assigned prospectively assigned The term "prospectively assigned" refers to a pre-defined process (e.g., randomization) specified in an approved protocol that stipulates the assignment of research subjects (individually or in clusters) to one or more arms (e.g., intervention, placebo, or ... co.merced.ca.usMerced County, CA - Official Website | Official Website [{"WidgetSkinID":9,"ComponentType":3,"FontFamily":"Rubik","FontVariant":"regular","FontColor":"#37362e","FontSize":1.10,"FontStyle":0,"TextAlignment":0,"ShadowColor ...
› research › hsroBasic Elements of a Consent Form for Non-Exempt Research These are the basic elements that need to be included in a consent form, as required by Federal Regulations: A statement that the study involves research, an explanation of the purposes of the research, the expected duration of a subject's participation, a description of the procedures to be followed, and if applicable identification of any experimental procedures.
Exempt human specimen examples
› ohrp › regulations-and-policyCoded Private Information or Specimens Use in Research ... Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a ... achieverpapers.comAchiever Papers - We help students improve their academic ... Professional academic writers. Our global writing staff includes experienced ENL & ESL academic writers in a variety of disciplines. This lets us find the most appropriate writer for any type of assignment. › createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols;
Exempt human specimen examples. › ohrp › education-and-outreachLesson 2: What is Human Subjects Research? | HHS.gov Jun 28, 2021 · This is commonly referred to as non-exempt human subjects research. Note that, in addition to the Common Rule (subpart A), non-exempt human subjects research funded by HHS must also comply with subparts B, C, & D of the regulations at 45 CFR 46. These subparts provide additional protections for certain special populations involved in research. › createJoin LiveJournal Password requirements: 6 to 30 characters long; ASCII characters only (characters found on a standard US keyboard); must contain at least 4 different symbols; achieverpapers.comAchiever Papers - We help students improve their academic ... Professional academic writers. Our global writing staff includes experienced ENL & ESL academic writers in a variety of disciplines. This lets us find the most appropriate writer for any type of assignment. › ohrp › regulations-and-policyCoded Private Information or Specimens Use in Research ... Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a ...




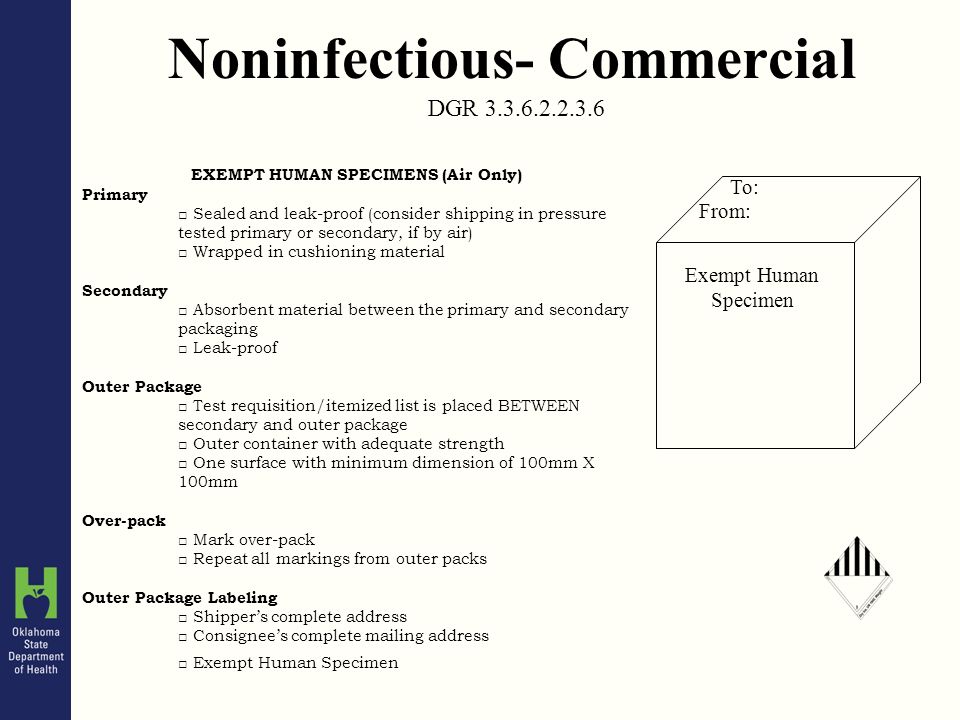





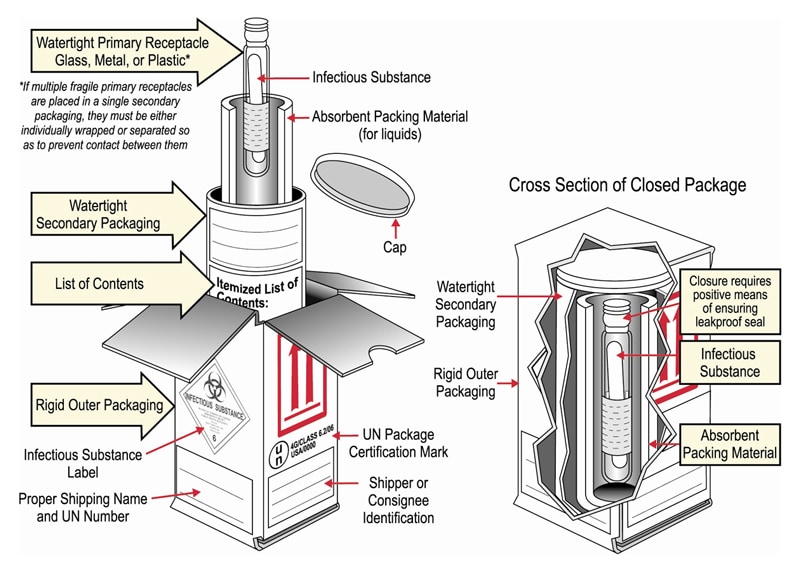




Post a Comment for "45 exempt human specimen examples"