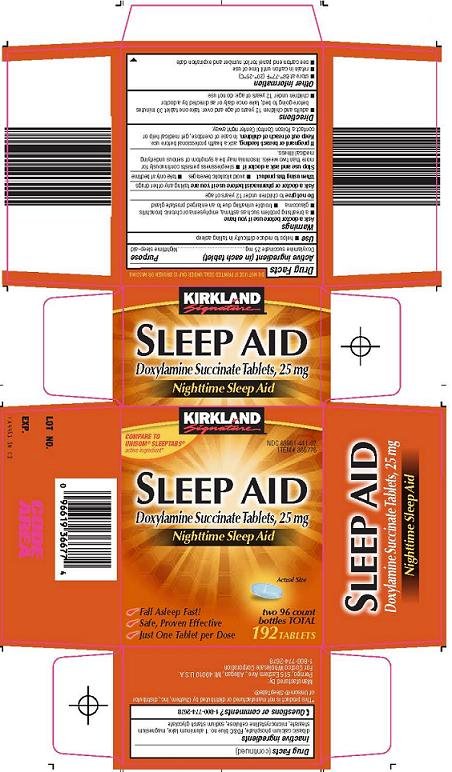
41 lot number on medication label
National Drug Code Directory | FDA The NDC Directory contains information on active and certified finished and unfinished drugs submitted to FDA in structured product labeling (SPL) electronic listing files by labelers. A labeler ... Quality System Regulation Labeling Requirements | FDA Various sections of the QS regulation have an impact on labeling: Section 21 CFR 820.80 (b) requires the inspection and testing of incoming materials including labeling; and 21 CFR 820.70 (f ...
The Over-the-Counter Medicine Label: Take a Look | FDA Many OTC medicines are sold in containers with child safety closures. Use them properly. Remember—keep all medicines out of the sight and reach of children. FDA. U.S. Food and Drug ...

Lot number on medication label
Guidance Document: Labelling of Pharmaceutical Drugs for Human Use The purpose of this document is to provide guidance to sponsors to facilitate compliance with the labelling requirements pursuant to sections 3, 9, and 10 of the Food and Drugs Act as well as related provisions of the Food and Drug Regulations, the Controlled Drugs and Substances Act, and its related Regulations including the Narcotic Control Regulations, Parts G and J of the Food and Drug ... What's on my medicine label? - Therapeutic Goods Administration (TGA) If the information you want is not on the label, consult a health professional such as a doctor or pharmacist. You can speak to an NPS MedicineWise. (link is external) pharmacist on 1300 134 237. You can also contact the sponsor or distributor of the medicine using the details on the label. FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) ... NDC Number Search: Active Ingredient Search: Application Number or Regulatory Citation Search: Company Search:
Lot number on medication label. What is a lot number and how do I identify it? - Acme United ... The lot number identifies when a product was manufactured. It will be in small type, all capital letters, and say "LOT #". On many products, it's on the back panel. On kit boxes, it's either on the top or bottom of the box, generally affixed to a label. Lot numbers have 2 parts: a letter, followed by a series of numbers. › cg › medication-safety-for-childrenMedication Safety for Children - What You Need to Know May 27, 2022 · Read the medicine label each time you give a medicine. Make sure you are giving your child the correct medicine. The label will list the correct amount and explain how to give it. Do not use any medicine that has expired or appears to have been tampered with. Ask your child's healthcare provider or a pharmacist to explain anything on the label ... National Drug Codes Explained: What You Need to Know What is a National Drug Code (NDC)? The NDC, or National Drug Code, is a unique 10-digit or 11-digit, 3-segment number, and a universal product identifier for human drugs in the United States. The 3 segments of the NDC identify: the labeler, the product, and the commercial package size. mattlaw.com › pharmacy-error-wrong-medicationPharmacy Error (When Pharmacist Gives Wrong Medication) she became severly tired sleeping almost 10 to 12 hors a day waking up in time to take her medication. upon checking her medication , the tablet was pink with the number 262 on it. the medication pantoprazole was for a stomach bacteria infection. the pink pill is used to treat schizophrenia. do she have any legal recourse. reply
Where is the lot number on a drug label? - AskingLot.com One may also ask, where is the lot number on a Walmart prescription bottle? Your prescription number is on the prescription label from the last fill. It's a 7-digit number located on the left side, near the top of the label. The expiration date is at the bottom of your prescription's label. It's usually listed as part of the available refills ... Amazon.com: Medication Labels - Recordkeeping & Labels: Industrial ... United Ad Label Medication Labels MULTIDOSE Vial Discard 28 Days After Opening, 1" Diameter, Permanent Paper Label, Fluorescent Yellow, One Roll of 885 Labels 5.0 out of 5 stars 3 $18.29 $ 18 . 29 ($18.29/Count) $22.34 $22.34 › health › conditions-treatmentsPfizer Recalls Additional Blood Pressure Medication - AARP Apr 26, 2022 · Advice to patients Patients who are taking Accupril should speak with their health care provider or pharmacy to determine whether they have the recalled product.. Those who have the recalled medication should contact Sedgwick, a claims-management company, toll-free at 888-345-0481, Monday through Friday, from 8 a.m. to 5 p. How to Understand FDA Lot Numbers | Healthfully Find your lot number. If you hear about a product recall and you have the product in your house, find the lot number, located on the packaging. The lot number may or may not be labeled as such; in other words, you may see "lot" followed by the number, but not always. When looking for the lot number, keep in mind that it will not be printed ...
How to Read Drug Labels - WebMD Directions. 5 /7. Check this part carefully. It tells you how much of the drug to take and how often to take it, called the dosage. For example, it may say to take two tablets every 4 to 6 hours ... Drug Recalls: What to Do and How to Find Your Medication Lot Number ... You can contact the FDA's Center for Drug Evaluation and Research (CDER) with any questions or concerns at druginfo@fda.hhs.gov or through the following toll-free numbers: 1-855-543-3784 or 1-310-796-3400. 2) Find your medication's lot number. Drug recalls pertain to certain lots of the medication that were made during a given time period. Lot Pill Images - Pill Identifier - Drugs.com How to use the pill identifier. Enter the imprint code that appears on the pill. Example: L484. Select the the pill color (optional). Select the shape (optional). Alternatively, search by drug name or NDC code using the fields above. NCBOP - Pharmacist FAQs Q. What information is required to be on the prescription label? A. The following information must be on every prescription label: 1. Name and address of the dispensing pharmacy. 2. Serial number of the prescription. 3. Date of the prescription. 4. Name of the prescriber. 5. Name of the patient. 6. Name and strength of the drug. 7.
Drug labelling - Wikipedia Drug labelling is also referred to as prescription labelling, is a written, printed or graphic matter upon any drugs or any of its container, or accompanying such a drug. Drug labels seek to identify drug contents and to state specific instructions or warnings for administration, storage and disposal. Since 1800s, legislation has been advocated ...
Prescription Drug Labeling Resources | FDA FDA's Prescription Drug Labeling Resources website provides over 150 labeling resources for the Prescribing Information, FDA-approved patient labeling, and/or carton and container labeling for ...
Code of Federal Regulations Title 21 - Food and Drug Administration The lot number on the label of a drug should be capable of yielding the complete manufacturing history of the package. An incorrect lot number may be regarded as causing the article to be misbranded. Sec. 201.19 Drugs; use of term "infant". The regulations affecting special dietary foods (§ 105.3(e) of this chapter) define an infant as a child ...
Lot number | definition of lot number by Medical dictionary lot number: An identifier assigned to a batch of medications. It facilitates drug manufacturing inventory control and tracing adverse incidents in a batch of contaminated medications. See also: number
Is NDC code required to appear on both 'bottle carton' and ... - Drugs.com As per the CFR regulation 21 Title CFR 201.2, "NDC number is requested but not required to appear on all drug labels and in all drug labeling, including the label of any prescription drug container furnished to a consumer." but our concern is specifically for the consistency of presence of NDC code on outer and inner labels for a bottle.
templatelab.com › medication-schedule40 Great Medication Schedule Templates (+Medication Calendars) The name of each medication; The dosage of each medication; What each of the medicines does You can find this information on the bottle label or package insert; Suggestions about how you will depict this information through pictures; The table will now serve as your guide in creating an outline of the data that you will include on your medicine ...
Safety Considerations for Container Labels and Carton Labeling to ... Designing Drug Label, Labeling, & Packaging ... -Lot number -Name of manufacturer, packer, or distributor • USP requires labels of official drug product to bear an expiration date
PDF Chapter 20 Labeling Medications and Expiration Dating Each container of medicinal drugs dispensed shall have a label or shall be accompanied by labeling. (1) Definitions. (a) "Controlled substance" means any substance named or described in Schedules II-V of Section 893.03, F.S. ... c. Lot number d. Strength of drug (Federal regulation) e. Dosage form f. Expiration Date 3. Small Containers a ...
Packaging and Labeling of Pharmaceutical Products Obtained from the ... For patients, the prescription container label may be the only source of instructions on how to take their medicines. In the United States, the legal requirements for a prescription label are set by federal law and state statutes. ... Lot Number (Source) Meets Label Standard a: Label Description: Meets Package Standard a: Package Description b ...


Post a Comment for "41 lot number on medication label"